A palladium/norbornene cooperative catalysis to access N-containing bridged scaffolds†
Abstract
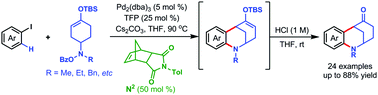
A palladium/norbornene cooperative catalysis promoted annulation involving an ortho-C–H amination and intramolecular Heck cascade between aryl iodides and functionalized amination reagents is reported, thereby providing a highly convergent access to the unique N-containing bridged scaffolds: hexahydro-2,6-methano-1-benzazocine. The salient features of the reaction include its broad substrate scope (with respect to aryl iodides), its high step economy, and good chemoselectivity. Preliminary studies underscore the future promise of rendering this Catellani-type annulation enantioselective.
- This article is part of the themed collection: 2019 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...