Multilayer graphene functionalized through thermal 1,3-dipolar cycloadditions with imino esters: a versatile platform for supported ligands in catalysis†
Abstract
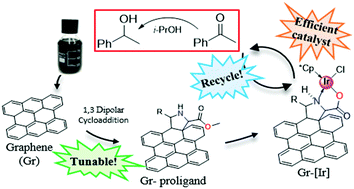
Multilayer graphene (MLG), obtained by mild sonication of graphite in NMP or pyridine, was fully characterized via atomic force microscopy (AFM), transmission electron microscopy (TEM), Raman spectroscopy and X-ray photoemission spectroscopy (XPS). Then, it was functionalized via 1,3-dipolar cycloaddition with azomethine ylides generated by thermal 1,2-prototropy of various imino esters. The resulting MLG, containing substituted proline-based amine functions, was characterized by XPS and it showed high nitrogen loading, ranging from 0.6 to 4.2 at% depending on the imino ester used. Among these functionalized MLGs a probe sample was subjected to ester hydrolysis and used as a heterogeneous N,O-chelating ligand to coordinate iridium atomic centers. This supported complex was also characterized by XPS and its catalytic activity was tested in the hydrogen transfer reduction of acetophenone, obtaining up to 85% yield. Furthermore, this catalyst could be recycled up to four times.


 Please wait while we load your content...
Please wait while we load your content...