Electrochemical oxidation of trivalent americium using a dipyrazinylpyridine modified ITO electrode†
Abstract
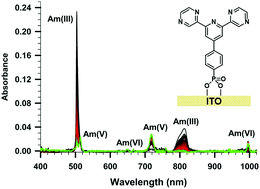
We present here the electrochemical oxidation of Am(III) to AmVO2+ and AmVIO22+ in pH 1 nitric acid using a mesoporous tin-doped indium oxide electrode modified with a covalently attached dipyrazinylpyridine ligand. The applied potential affects the distribution of Am oxidation products. At potential 1.8 V, only Am(V) is observed, while increasing the potential to as much as 2.0 V, results in oxidation of Am(III) to Am(V) and subsequent oxidation of Am(V) to Am(VI). At applied potentials >2.0 V, Am(III) is oxidized to Am(V), while Am(VI) is reduced to Am(V). The latter reduction reaction is likely due to the increased rate of hydrogen peroxide formation from the 2-electron oxidation of water at the electrode at these high potentials. The development of future ligand modified electrodes for actinide oxidations must consider how they facilitate Am oxidations while disfavoring unwanted or competing reactions.
- This article is part of the themed collection: New molecules and materials from the f-block

 Please wait while we load your content...
Please wait while we load your content...