Electrochemical benzylic oxidation of C–H bonds†
Abstract
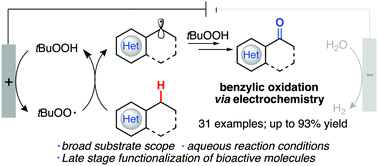
Oxidized products have become increasingly valuable as building blocks for a wide variety of different processes and fine chemistry, especially in the benzylic position. We report herein a sustainable protocol for this transformation through C–H functionalization and is performed using electrochemistry as the main power source and tert-butyl hydroperoxide as the radical source for the C–H abstraction. The temperature conditions reported here do not increase above 50 °C and use an aqueous-based medium. A broad substrate scope is explored, along with bioactive molecules, to give comparable and increased product yields when compared to prior reported literature without the use of electrochemistry.


 Please wait while we load your content...
Please wait while we load your content...