Monitoring compositional changes in Ni(OH)2 electrocatalysts employed in the oxygen evolution reaction†
Abstract
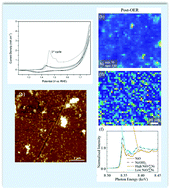
Electrochemical water splitting to generate hydrogen has been identified as a possible solution to the storage of intermittent renewable energy. However there are still challenges remaining in the development of stable electrocatalysts for the oxygen evolution half-reaction. Here we investigate the effects that the oxygen evolution reaction (OER) has on an electrodeposited Ni(OH)2 catalyst operated under alkaline conditions. The electrocatalyst was characterised by established methods including cyclic voltammetry, electrochemical impedance spectroscopy, scanning electron microscopy, atomic force microscopy, X-ray photoelectron spectroscopy both before and after the OER to identify changes that may have occurred in the structure and/or composition of the catalyst. In addition, synchrotron X-ray absorption near edge structure mapping was used to generate spatially resolved maps of the species present within the Ni(OH)2 catalyst and how they change in a heterogeneous manner into a NiO species after the OER. When compared to the morphological data it suggests that changes in the morphology after the OER can be correlated to the formation of NiO within the newly formed clusters that were generated across the electrocatalyst.
- This article is part of the themed collection: Versatile Electrochemical Approaches


 Please wait while we load your content...
Please wait while we load your content...