Mixed-linker MOFs with CAU-10 structure: synthesis and gas sorption characteristics†
Abstract
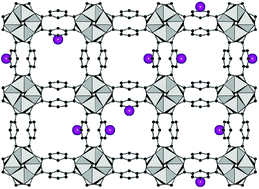
The metal–organic framework compound [Al(OH)(BDC-Br)] (1) (BDC-Br2− = 5-bromo-1,3-benzenedicarboxylate) denoted CAU-10-Br was synthesised under solvothermal reaction conditions. Its structure was successfully refined by Rietveld methods. The framework is based on the connection of infinite helical chains of cis-corner-sharing AlO6-polyhedra via BDC-Br2− ions. Thus non-intersecting parallel channels are formed, each periodically varying in diameter between 1.1 and 6.6 Å. Nevertheless 1 adsorbs CO2 at 298 K, while it is non-porous towards H2 and N2 at 77 K. Employing high-throughput (HT) methods we identified synthesis conditions that lead to the formation of mixed-linker MOFs with CAU-10 topology. Starting with a molar ratio H2BDC : H2BDC-Br = 3 : 1 we established a synthesis procedure for the partially bromo-functionalised mixed-linker-MOF [Al(OH)(BDC)0.8(BDC-Br)0.2] (2) denoted as CAU-10-H/Br. Starting with a molar ratio H2BDC-NH2 : H2BDC-NO2 = 1 : 1 the partially NO2- and NH2-functionalised mixed-linker MOF [Al(OH)(BDC-NO2)0.55(BDC-NH2)0.23(BDC-NHCHO)0.22] (3) denoted as CAU-10-NO2/NH2 was synthesised, in which the NH2-groups were found to be partially formylated. The partial bromo-functionalisation in CAU-10-H/Br (2) leads to a lower sorption capacity in comparison with the parent structure CAU-10-H, while the pore accessibility is comparable. The incorporation of NO2-, NH2- and NHCHO-groups in CAU-10-NO2/NH2 (3) does not only affect the accessibility of the pores for N2, but results also in an increased capacity for H2 and CO2 in comparison with the parent structure CAU-10-NO2. The reproducibility of the synthesis procedures was tested regarding the composition of the MOFs and the resulting gas

 Please wait while we load your content...
Please wait while we load your content...