Unraveling the effect of ZrO2 modifiers on the nature of active sites on AuRu/ZrO2 catalysts for furfural hydrogenation†
Abstract
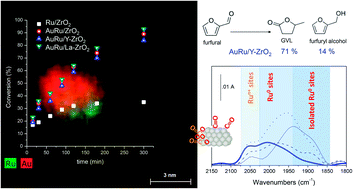
Ru and AuRu nanoparticles were prepared by a sol-immobilization methodology and deposited on different doped zirconia supports (ZrO2, Y–ZrO2 and La–ZrO2). The catalysts were characterized by analytic transmission electron microscopy (TEM), Fourier transform infrared spectroscopy (FTIR) in a controlled atmosphere and X-ray photoelectron spectroscopy (XPS). TEM analysis showed that AuRu catalysts consist of Au–Ru particle aggregates with small Ru particles enriched on the Au surface. FTIR experiments of adsorbed CO and XPS analyses revealed that the presence of gold modifies the electronic properties of Ru, confirming the bimetallic nature of AuRu nanoparticles. The catalysts were tested in furfural hydrogenation using isopropanol as the hydrogen donor. The addition of Au to Ru did not significantly modify the activity and selectivity but enhanced the resistance to deactivation. The acid–base properties were monitored by acetonitrile adsorption followed by FTIR spectroscopy. It was shown that the acidity of the support greatly influences the selectivity. In particular, 71% selectivity to gamma-valerolactone was achieved over AuRu/Y–ZrO2, due to the modified acidic strength of Zr4+ sites on the Y-doped oxide.


 Please wait while we load your content...
Please wait while we load your content...