Rational design and synthesis of yellow-light emitting triazole fluorophores with AIE and mechanochromic properties†
Abstract
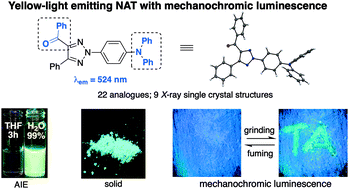
Previously, we reported that N-2-aryl triazoles (NATs) exhibited good fluorescence activity in the UV/blue light range. In an effort to achieve biocompetitive NAT fluorophores with green/yellow emission, a new class of 4-keto-2-(4′-N,N-diphenyl)-phenyl triazoles were designed and synthesized. Herein, we present our study on these novel fluorophores which demonstrated excellent luminescence emission both in solution (Φ up to 96%) and in the solid state (Φ up to 43%). Furthermore, these new compounds showed aggregation-induced emission (AIE) properties and reversible mechanochromic luminescence properties, which suggested their potential applications in chemical and materials science.


 Please wait while we load your content...
Please wait while we load your content...
