Removal of pharmaceuticals and personal care products (PPCPs) from water and wastewater using novel sulfonic acid (–SO3H) functionalized covalent organic frameworks†
Abstract
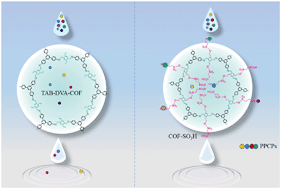
This study describes a promising porous and efficient adsorbent, a sulfonic acid (–SO3H) functionalized covalent organic framework (COF-SO3H), for the removal of pharmaceuticals and personal care products (PPCPs) in water and wastewater. COF-SO3H was prepared through introducing sulfonic acid polar functionalities via a facile solvothermal synthesis method. Studies have shown that COF-SO3H has a strong affinity for the thirteen drugs investigated in this study, especially for diclofenac (DCF). The maximum adsorption capacity for DCF was 770 mg g−1, which amounted to 9.3 times that of commercial AC, 1.5 times that of graphene oxide (GO), and 3 times that of 18% SO3-UiO-66. Further discoveries were that the adsorption isotherm data conformed well with the Langmuir model. It was also found that adsorption of DCF follows the pseudo-second-order kinetic model and thermodynamic values, ΔH < 0 and ΔG < 0, showed the exothermic nature and spontaneity of the adsorption process. Additionally, the adsorption mechanisms observed in this experiment, such as π–π interactions and the formation of hydrogen bonds, were also verified through various characterization methods. The cyclic regeneration of adsorbents and the effects of water quality constituents were estimated to assess performance in actual water/wastewater systems. Ultimately, the overall results of this study emphasized the suitability of the COF-SO3H adsorbent to remove micropollutants from real water and wastewater systems.


 Please wait while we load your content...
Please wait while we load your content...