Deficit in the epidermal barrier induces toxicity and translocation of PEG modified graphene oxide in nematodes†
Abstract
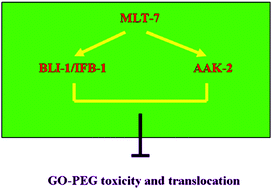
The developmental basis for the epidermal barrier against the translocation of nanomaterials is still largely unclear in organisms. We here investigated the effect of deficits in the epidermal barrier on the translocation and toxicity of PEG modified graphene oxide (GO-PEG) in Caenorhabditis elegans. In wild-type or NR222 nematodes, GO-PEG exposure did not cause toxicity and affect the expression of epidermal-development related genes. However, GO-PEG exposure resulted in toxicity in mlt-7(RNAi) nematodes with deficit in the function of epidermal barrier. Epidermal RNAi knockdown of mlt-7 allowed GO-PEG accumulation and translocation into targeted organs through the epidermal barrier. Epidermal-development related proteins of BLI-1 and IFB-1 were identified as targets for MLT-7 in the regulation of GO-PEG toxicity and accounted for MLT-7 function in maintaining the epidermal barrier. AAK-2, a catalytic α subunit of AMP-activated protein kinase, was identified as another target for MLT-7 in the regulation of GO-PEG toxicity. AAK-2 functioned synergistically with BLI-1 or IFB-1 in the regulation of GO-PEG toxicity. Our data provide the molecular basis for the role of epidermal barrier against the toxicity and translocation of nanomaterials in organisms.
- This article is part of the themed collection: Toxicology Research Recent HOT articles


 Please wait while we load your content...
Please wait while we load your content...