N-Cyanoimine as an electron-withdrawing functional group for organic semiconductors: example of dihydroindacenodithiophene positional isomers†
Abstract
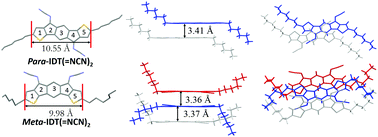
Manipulating frontier molecular orbitals by chemical design is one of the chief aspects of organic electronics. For applications in the field of n-type organic field-effect transistors (OFET), depressing the LUMO energy level is particularly important to ensure efficient charge injection. In this work, we report the incorporation of electron-withdrawing cyanoimine functional groups on the bridges of dihydroindacenodithiophene regioisomers. Cyanoimines are barely known in the field of organic electronics but herein have been found to be highly efficient in depressing the LUMO energy level of an organic semiconductor and can thus be envisaged as an attractive alternative to widely known electron-withdrawing units such as dicyanovinylene. This work focuses on a detailed structure–property relationship study, including the incorporation in n-type OFETs, of two dihydroindacenodithiophene regioisomers bearing two cyanoimine groups either in a syn- or an anti-configuration. As far as we know, this work is only the second example reported to date on N-cyanoimines incorporated in n-type OFETs, and shows the potential of these functional groups in organic electronics.
- This article is part of the themed collection: Journal of Materials Chemistry C Advisory Board Collection


 Please wait while we load your content...
Please wait while we load your content...