Microwave-assisted synthesis of cyclen functional carbon dots to construct a ratiometric fluorescent probe for tetracycline detection†
Abstract
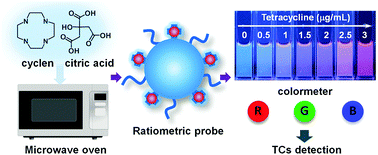
Carbon dots (CDs) prepared by the crosslinking and carbonization reaction between the carboxyl and amino groups of organic molecules have the characteristics of high fluorescence quantum yield and tunable emission colors, enabling their wide use in chemical sensing and bioimaging. However, straight-chain organic amines are mostly used in the preparation of carbon dots. Here, we chose cyclen that contained an azamacrocyclic ring and citric acid to synthesize bright blue-emitting fluorescent CDs by the microwave method. Under 750 W microwave irradiation, the condensation–crosslinking–carbonization process can be completed within 5 minutes with a quantum yield of up to 27.6%. Taking the advantage of using cyclen, the resulting CDs still have a ring-like structure on the surface, and thus can coordinate with Eu3+ ions to construct a ratiometric fluorescent probe for tetracycline detection. When tetracycline is present in the detection system, a CD–Eu3+–tetracycline ternary complex is formed and generates an intense red emission of Eu3+. Meanwhile, the blue emission of the CDs remains unchanged, realizing accurate tetracycline detection by recording the red to blue emission ratio. Besides, the output color changes from blue to red upon increasing the concentration of tetracycline. Therefore, a smartphone based tetracycline detection technique was successfully developed through the corresponding RGB value analysis.
- This article is part of the themed collection: International Year of the Periodic Table : Low Dimensional Carbon Systems


 Please wait while we load your content...
Please wait while we load your content...