Solution-processed N-trialkylated triindoles for organic field effect transistors†
Abstract
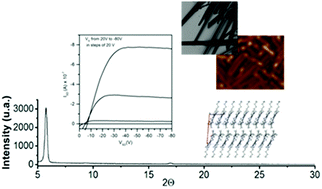
Three crystalline N-trialkyltriindoles in which the length of the alkyl chains attached to the nitrogen has been enlarged from a methyl to a butyl and to a hexyl group have been investigated in the search for semiconducting triindole easy to process from solution. We have found that the number of carbon atoms of the N-alkyl chains has a significant impact on how these molecules interact with each other in the bulk materials and it strongly influences the final morphology of the crystals, which grow as long crystalline wires, cubes or microbelts. Single crystal analysis allows us to recognize the contribution of several cooperative CH–π interactions to guide the self-assembly of these types of molecules. In addition, alkyl chain engineering allows triindole derivatives processable for solution, which render OFETs showing field effect mobilities of 0.03 cm2 V−1 s−1 and 6 × 10−3 cm2 V−1 s−1 when vapor deposited and drop casted, respectively. The results of this study represent a step forward towards the rational control of the supramolecular arrangement of this high performance semiconducting platform, this being a key fact for designing efficient solution-processed organic semiconductors.


 Please wait while we load your content...
Please wait while we load your content...