Superpermeable nanoporous carbon-based catalytic membranes for electro-Fenton driven high-efficiency water treatment†
Abstract
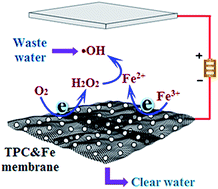
Based on the Hagen–Poiseuille equation, theoretical membrane permeability is inversely and directly proportional to its thickness and pore-radius, respectively, indicating that a thin membrane with an effective pore-radius is extremely permeable. However, the trade-off between selectivity and permeability restricts their applications for pressure-driven separation membranes. Hence, balancing the contradiction between the permeability and selectivity of a separation membrane is desired in future applications. Herein, we report a superpermeable nanoporous carbon (PC) membrane which offers a separation layer (∼80 nm) and effective pore radius (∼20 nm) by selectively removing parts of carbon atoms, thus greatly decreasing the transport resistance of water molecules through the membrane. The experimental results have shown a water flux of ∼8000 L m−2 h−1 bar−1 for the membrane. High selectivity is demonstrated via selective separation of nanoparticles with their narrowly distributed pores. Self-production of H2O2 and self-circulation of Fe2+/Fe3+ was achieved simultaneously on the PC membrane via coupling with electro-Fenton technology, which leads to the in situ production of hydroxyl radicals. The removal efficiencies of organic contaminants (SMX, BPA and phenol) by electro-Fenton driven PC membranes were ∼96.3%, ∼97.4% and 92.1%, respectively. Given their compatible high permeability and high selectivity, our porous PC membranes might provide an alternative as future membranes and motivate the design of innovative membranes.


 Please wait while we load your content...
Please wait while we load your content...