Linking surface chemistry to photovoltage in Sr-substituted LaFeO3 for water oxidation†
Abstract
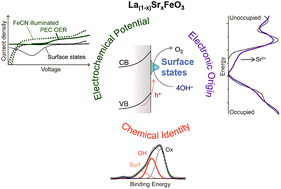
Perovskite oxides are promising materials for photoabsorbers and electrocatalysts for solar-driven water oxidation. Aliovalent doping can tune the transition metal redox properties as well as the material band gap and conductivity, resulting in a rich phase space of possible water-oxidation photoanodes. We consider the substitution of Sr2+ for La3+ in LaFeO3 epitaxial thin films, where the well-defined nature of the flat surface and path for charge transport enables fundamental studies of photoelectrochemical (PEC) water oxidation. Sr incorporation increases the photovoltage, but at the expense of photocurrent. The use of a fast redox couple to efficiently collect photogenerated holes indicates an even lower onset of oxidative current; the difference between this onset and that of water oxidation arises from the involvement of surface states during water oxidation. Measurements of the surface speciation in a humid environment demonstrate that Sr incorporation also promotes hydroxylation, suggesting that water oxidation proceeds through the oxidation of FeIII–OH states, which is facilitated by Sr incorporation and results in an increased photovoltage.
- This article is part of the themed collection: Journal of Materials Chemistry A Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...