Chemical-assisted hydrogen electrocatalytic evolution reaction (CAHER)
Abstract
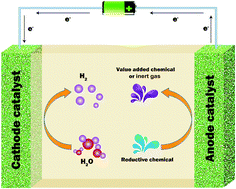
Hydrogen energy, which is environmentally friendly and sustainable, has attracted significant attention from researchers worldwide for its promising future applications. Water electrolysis in conjunction with renewable electricity (from wind, photovoltaics, tides, etc.) can provide a “zero emission” source of hydrogen. However, water electrolysis is severely hampered by its slow kinetics, especially that of the oxygen evolution reaction (OER) at the cathode. Recently, an alternative strategy for water splitting, the chemical-assisted hydrogen electrocatalytic evolution reaction (CAHER), has been developed and shows promising activity. This strategy enables the substitution of a relatively facile chemical oxidation reaction (COR) for the sluggish cathodic OER. The COR is not only kinetically faster, but also can produce high value products. Efforts have been made to identify suitable chemicals and correspondingly efficient catalysts. Understanding the mechanisms of CAHER is highly beneficial for system design, chemical selection and catalyst optimization. Therefore, the most recent progress in CAHER is introduced in this mini-review from the aspects of basic principles, the diverse auxiliary reductive chemicals and the development of electrocatalysts. Possible mechanisms for the hydrogen evolution at the cathode and oxidation/degradation of chemicals at the anode are proposed to elucidate the CAHER process. Finally, promising strategies for improving CAHER performance with regard to future developments are outlined.
- This article is part of the themed collection: Recent Review Articles


 Please wait while we load your content...
Please wait while we load your content...