Hydrogenation of fourteen biomass-derived phenolics in water and in methanol: their distinct reaction behaviours†
Abstract
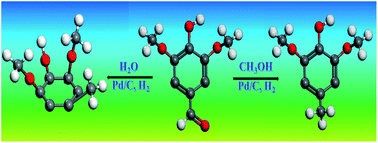
Phenolic compounds are an important component of pyrolysis oil (bio-oil). Understanding their reaction behaviours during hydrogenation helps to clarify the reaction network during hydrotreatment of bio-oil. This study investigated the hydrogenation of typical phenolic compounds in bio-oil in water, which is an important fraction of bio-oil, and in methanol, which is used to esterify bio-oil. The results indicated that hydrogenation of the phenolics is more efficient in water than in methanol. With water medium, both the unsaturated oxygen-containing functionalities and the benzene ring of the phenolics can be effectively hydrogenated. In comparison, with methanol medium, only the unsaturated oxygen-containing functionality can be hydrogenated, while the hydrogenation of the benzene ring is rather difficult. Stereoselectivity was also observed in the hydrogenation of phenolics with three or more hydroxyl groups/methoxyl groups. For phenolics with two hydroxyl groups/methoxyl groups, the hydrogenation produces both cis- and trans-configurations.


 Please wait while we load your content...
Please wait while we load your content...