The hexameric resorcinarene capsule as an artificial enzyme: ruling the regio and stereochemistry of a 1,3-dipolar cycloaddition between nitrones and unsaturated aldehydes†
Abstract
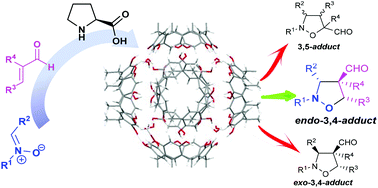
When a 1,3-dipolar cycloaddition between nitrones and α,β-unsaturated aldehydes catalyzed by L-proline derivatives takes place inside the hexameric resorcin[4]arene capsule the products are obtained in higher yields and selectivity, with respect to the reaction in bulk solution. The 4-formyl regioisomers were obtained preferentially, with an excellent preference for the endo-diastereoisomers and with moderate to good enantioselectivities. An extended in silico study was conducted to rationalize the observed outcome and to investigate the equilibria between the reagents outside and inside the capsule.
- This article is part of the themed collections: Organic Chemistry Frontiers HOT articles for 2018 and Organic Chemistry Frontiers HOT articles for 2017


 Please wait while we load your content...
Please wait while we load your content...