Catalytic asymmetric synthesis of pyrrolidine derivatives bearing heteroatom-substituted quaternary stereocenters†
Abstract
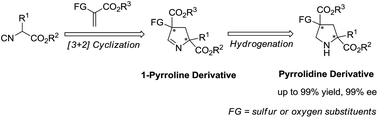
An enantioselective [3 + 2] cycloaddition of heteroatom-substituted alkenes with α-substituted isocyanoacetates has been developed. The resulting cycloadducts could be further transformed to access a number of structurally diverse and biologically important pyrrolidine derivatives which bear sulfur- or oxygen-substituted quaternary stereocenters. Excellent reactivity and enantioselectivity were obtained for a broad range of substrates.


 Please wait while we load your content...
Please wait while we load your content...