Aluminium-catalyzed terpolymerization of furfuryl glycidyl ether with epichlorohydrin and ethylene oxide: synthesis of thermoreversible polyepichlorohydrin elastomers with furan/maleimide covalent crosslinks†
Abstract
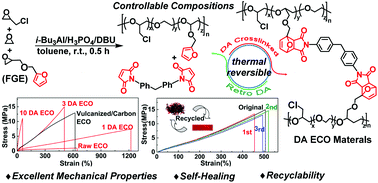
By using an alkylaluminium catalyst system i-Bu3Al/H3PO4/1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), the random copolymerization and terpolymerization of furfuryl glycidyl ether (FGE) with epichlorohydrin (ECH) and ethylene oxide (EO) have been achieved for the first time to afford a new family of furan functionalized polyepichlorohydrin elastomers. The composition of the obtained FGE/ECH copolymers, FGE/EO copolymers and FGE/ECH/EO terpolymers coincided with the co-monomer feed ratio, and furan groups could be quantitatively and randomly incorporated into polyepichlorohydrin elastomers. Based on the Diels–Alder (DA) reaction of furan functionalized polyepichlorohydrin elastomers and 4,4′-methylenebis(N-phenylmaleimide), a new series of thermoreversible cross-linked polyepichlorohydrin elastomers was successfully synthesized. These thermoreversible DA cross-linked polyepichlorohydrin elastomers possess mechanical properties that can be tuned by adjusting the content of FGE units in the FGE/ECH/EO terpolymers. The mechanical properties of DA cross-linked polyepichlorohydrin elastomers were significantly better than those of traditional vulcanized polyepichlorohydrin elastomers, and they could be used as a structural material without reinforcing agents. More importantly, the DA cross-linked polyepichlorohydrin elastomers could be remolded and self-healed without loss of performance.


 Please wait while we load your content...
Please wait while we load your content...