Reactive cyclic intermediates in the ProTide prodrugs activation: trapping the elusive pentavalent phosphorane†‡
Abstract
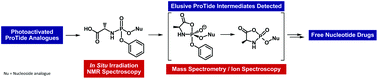
Nucleotide prodrugs (ProTides) based on phosphate or phosphonate compounds are potent and successfully marketed antiviral drugs. Although their biological properties are well explored, experimental evidence on the mechanism of their activation pathway is still missing. In this study, we synthesized two ProTide analogues, which can be activated by UV light. Using 31P and 13C NMR spectroscopy with in situ irradiation, we followed the ProTide activation pathway in various solvents, and we detected the first proposed intermediate and the monoamidate product. Furthermore, we used mass spectrometry (MS) coupled with infrared spectroscopy in the gas phase to detect and to characterize the elusive cyclic pentavalent phosphorane and cyclic acyl phosphoramidate intermediates. Our combined NMR and MS data provided the first experimental evidence of the cyclic intermediates in the activation pathway of ProTide prodrugs.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...