Anti-inflammatory fusicoccane-type diterpenoids from the phytopathogenic fungus Alternaria brassicicola†
Abstract
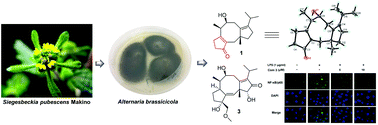
Altering the cultivation conditions, such as temperature, media compositions, illumination, aeration, the shape of the culturing flask, etc., is regarded as a useful strategy for the exploitation of structurally novel and bioactive secondary metabolites, which inspired us to explore the chemical and pharmacological diversities from the genetically powerful fungus Alternaria brassicicola. As a result, twelve fusicoccane-type diterpenoids, including eight new ones, termed brassicicenes Q–X (1–8), were isolated from a modified rice medium. Biosynthetically, all these compounds were derived from the mevalonic acid (MVA) pathway, and their structures incorporating absolute configurations were assigned by the interpretation of spectroscopic (HRESIMS and 1D and 2D NMR) analyses, chemical conversion, a modified Mosher's method, 13C NMR calculation, and single-crystal X-ray diffraction (Cu Kα). Structurally, compound 1 was an unusual 16-nor-dicyclopenta[a,d]cyclooctane diterpenoid bearing a fused cyclopent-2-en-1-one moiety. In addition, compound 3 was found to show significant anti-inflammatory activity against the production of NO, TNF-α, and IL-1β at 10 μM. Further western blot and immunofluorescence experiments found the mechanism of action to be that 3 inhibited the NF-κB-activated pathway, highlighting it as a promising starting point for the development of new anti-inflammatory agents.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...