Transition-metal-free KI-catalyzed regioselective sulfenylation of 4-anilinocoumarins using Bunte salts†
Abstract
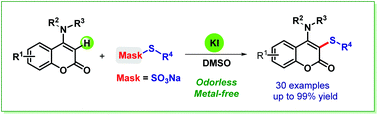
An efficient and eco-friendly protocol for the KI-catalyzed regioselective sulfenylation of 4-anilinocoumarins with Bunte salts was established. The reaction worked smoothly under metal-free conditions and afforded a wide range of sulfenylated 4-anilinocoumarins with potential bioactivity in moderate to excellent yields. This method would expand the still limited scope of synthesis methods for C–S bond formation using Bunte salts.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...