The role of proton shuttling mechanisms in solvent-free and catalyst-free acetalization reactions of imines†
Abstract
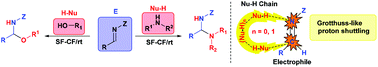
Proton transfer is central to the understanding of chemical processes. More so in addition reactions of the type NuH + E → Nu–EH taking place under solvent-free and catalyst-free conditions. Herein we show that the addition of alcohols or amines (the NuH component) to imine derivatives (the E component), in 1 : 1 ratio, under solvent-free and catalyst-free conditions, are efficient methods to access N,O and N,N-acetal derivatives. In addition, computational studies reveal that they are catalyzed reactions involving two or even three NuH molecules operating in a cooperative manner as H-bonded NuH⋯(NuH)n⋯NuH associates (many body effects) in the transition state through a concerted proton shuttling mechanism (addition of alcohols) or stepwise proton shuttling mechanism (addition of amines), thereby facilitating the key proton transfer step.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...