Palladium-catalyzed selective synthesis of 3,4-dihydroquinazolines from electron-rich arylamines, electron-poor arylamines and glyoxalates†
Abstract
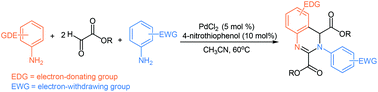
A simple palladium-catalyzed selective synthesis of structurally diverse 3,4-dihydroquinazolines from electron-rich arylamines, electron-poor arylamines and glyoxalates has been developed under mild conditions. This reaction is carried out in a tandem manner constituted by the condensation of arylamines and glyoxalates, the selective Diels–Alder cycloaddition and oxidation processes, in which 4-nitrothiophenol was used as the key ligand.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...