Supramolecular chiroptical switching of helical-sense preferences through the two-way intramolecular transmission of a single chiral source†
Abstract
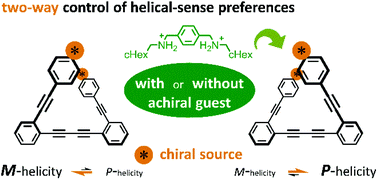
We demonstrate a chiroptical switching system with a simple molecule. The molecule contains a pair of chromophores of diphenylacetylene that are linked with a diyne bond and arranged to exert exciton coupling in helically folded forms with (M)- or (P)-helicity. A tertiary amide group is attached to each end of the looped molecule. The amide carbonyls were used to capture a ditopic hydrogen-bonding guest. A chiral auxiliary group on the amide nitrogen acted as a chiral handle to control the helical-sense preference of dynamic helical forms of the loop. The helical-sense preference is brought about by an intramolecular transmission of point chirality associated with the loop. The preferred sense was switched upon complexation with an achiral additive through the formation of hydrogen bonds. In both states, before and after complexation, the helical-sense preferences were controlled through two-way transmission of the single chiral source.
- This article is part of the themed collection: Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...