E–Z isomerization in Suzuki cross-couplings of haloenones: ligand effects and evidence for a separate catalytic cycle†
Abstract
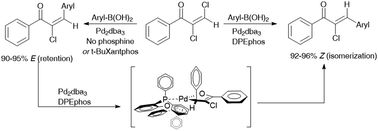
Suzuki cross-coupling of haloalkenes is generally assumed to occur with retention of the alkene stereochemistry. While studying Suzuki cross-couplings on E-1,2-dichlorovinyl phenyl ketone, we were surprised to observe extensive isomerization. More surprisingly, the ligand employed strongly influenced the degree of isomerization: DPEphos and Xantphos led to 96% isomerized cross-coupled product whereas reactions in the absence of a phosphine ligand, or reactions employing t-BuXantphos, gave 94% retention of stereochemistry. While E–Z isomerization in Pd-catalyzed vinylic couplings has previously been attributed to events within the cross-coupling catalytic cycle, we present experimental and computational evidence for a separate Pd-catalyzed isomerization process in these reactions.


 Please wait while we load your content...
Please wait while we load your content...