Ligand-driven formation of halogen bonds involving Au(i) complexes†
Abstract
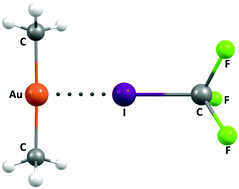
In a theoretical investigation at various levels of theory we show that even gold in the oxidation state +1 (i.e. formally positively charged) can behave as a Lewis base and, as a result, a halogen bond acceptor. Depending on the nature of the ligands in the gold complex the resultant halogen bonds are of similar strength to that found in the triiodide ion, but weaker than those involving the auride ion (highest value −59.4 kcal mol−1 for Au−·I2). The strength of the halogen bonds involving I2 range from −46.3 kcal mol−1 for the anionic adduct [((Me)2N)2Au]−·I2 to −5.9 kcal mol−1 for the cationic [(H3N)2Au]+·I2 adduct (calculated at the MP2/aug-cc-pVTZ-pp level of theory), and still weaker for adducts involving poorer halogen bond donors.
- This article is part of the themed collection: The halogen bond: a new avenue in recognition and self-assembly


 Please wait while we load your content...
Please wait while we load your content...