Cooperative halogen bonding and polarized π-stacking in the formation of coloured charge-transfer co-crystals†
Abstract
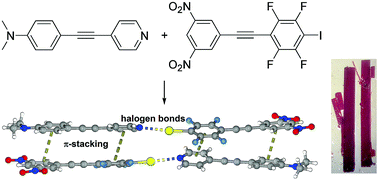
Red co-crystals are formed between the matched complementary electron rich halogen bond acceptors, isomeric 4-(N,N-dimethylamino)phenylethynylpyridines and the electron poor halogen bond donor, 1-(3,5-dinitrophenylethynyl)-2,3,5,6-tetrafluoro-4-iodobenzene. The red 1 : 1 cocrystals exhibit strong halogen bonding and strong π-stacking. The N⋯I distances range from 2.80 to 2.85 Å and the C–I⋯N angles are between 169.9 and 175.8°. In all four structures the donor and acceptor molecules are alternately π-stacked with the centroid to centroid distances between the dinitrophenyl moiety and the dimethylaminophenyl moiety ranging from 3.61 to 3.73 Å. The calculated π–π stacking binding energy is −22.24 kcal mol−1 for the complex between 4-[4-(N,N-dimethylamino)phenylethynyl]pyridine and 1-(3,5-dinitrophenylethynyl)-2,3,5,6-tetrafluoro-4-iodobenzene while the calculated halogen bond binding energy between the same couple is −7.97 kcal mol−1.
- This article is part of the themed collection: The halogen bond: a new avenue in recognition and self-assembly


 Please wait while we load your content...
Please wait while we load your content...
