Half-sandwich type rhodium(iii)–aminohydroxamate complexes: the role of the position of the amino group in metal ion binding†‡
Abstract
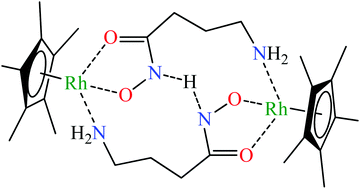
Complex formation equilibria between [(η5-Cp*)RhIII(H2O)3]2+ and aminohydroxamic acids (L-2-amino-N-hydroxyacetamide (α-alahaH), 3-amino-N-hydroxypropanamide (β-alahaH) and 4-amino-N-hydroxybutanamide (GABAha, γ-abhaH)) having the primary amino group in different chelatable positions relative to the hydroxamic function were studied using pH-potentiometric, 1H NMR and ESI-MS methods and the formation constants of the complexes present in aqueous solution are reported. The relative order of the pH-dependent conditional stability of the hydroxamate type (O,O) and (Namino,Nhydroxamato) chelates was found to determine to a great extent the coordination modes both in the mono- and various dinuclear species formed. While with α-alaha−, a 5-membered (N,N) chelated mononuclear complex predominates, with β-alaha− in a wide pH-range, very stable dinuclear cluster ions exist. With γ-abha−, in the most stable complexes, two ligands (in reverse variation) link two half-sandwich cations, coordinating each ligand via the hydroxamate chelate to one metal centre, while via the amino-N to the other one. This arrangement seems to be further stabilized by a hydrogen bond as DFT calculations support the extra stabilization effect of internal H-bonding in [{(η5-Cp*)RhIII}2H−1(γ-abha)2]+. The synthesis, spectral (NMR and IR) and MS characterization of a novel complex with an iridium analogue, [(η5-Cp*)IrIII(α-alaha)Br] (1) is also described. This complex was tested for its in vitro cytotoxicity using human-derived cancer cell lines (A2780, HeLa, DU-145, A549, and MCF-7) and showed insignificant anti-proliferative activity in the micromolar concentration range.
- This article is part of the themed collection: Equilibrium Solution Coordination Chemistry


 Please wait while we load your content...
Please wait while we load your content...