Orbital control over the metal vs. ligand reduction in a series of neutral and cationic bis(cyclopentadienyl) Ti(iv) complexes†
Abstract
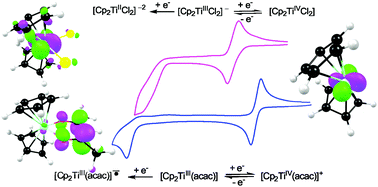
Quantum chemistry calculations showed that the first reduction, as experimentally observed by cyclic voltammetry for a series of neutral and cationic functionalised bis(cyclopentadienyl) titanium(IV) derivatives, is metal based, involving the chemically and electrochemically reversible TiIV/TiIII redox couple. The second reduction peak observed is chemically and electrochemically irreversible. For the neutral [Cp2TiIV(ligand)n] (n = 1 or 2) complexes (Cp = cyclopentadienyl), the second irreversible reduction peak is metal-based, while for the cationic [Cp2TiIV(ligand)]+ complexes, the second irreversible reduction peak involves ligand reduction, leading to a TiIII species coupled to a ligand radical. The electronic properties of the substituents directly influence electron density at the reduction centre when π-interactions between the electro-active centre and the substituent groups exist in the lowest unoccupied molecular orbital (LUMO) of the complexes. In this case, a number of relationships between the electronic properties of the substituents and the reduction potentials have been affirmed, yielding linear relationship between experimentally measured reduction potentials and DFT calculated energies (energy of the LUMO, electron affinity, Mulliken electronegativity and global electrophilicity index). However, the substituent effect is ill defined when the LUMO is highly localised without π-interaction between the electro-active centre and the substituents.


 Please wait while we load your content...
Please wait while we load your content...