Nanodiamond-based non-canonical autophagy inhibitor synergistically induces cell death in oxygen-deprived tumors†
Abstract
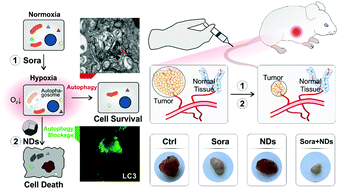
The tumor microenvironment (TME) plays a key role in tumor progression and has been actively explored as a promising target for cancer therapy. One key challenge in TME-based therapy lies in the presence of high levels of autophagy under oxygen-deprived conditions (referred to as hypoxia), which is an evolution-based adaptive strategy of cancer cells to facilitate tumor growth and metastasis. Here, we utilize a type of biocompatible nanoparticle, nanodiamonds (NDs), to block autophagic flux in cultured cells and xenograft tumors. Whereas ND treatment has little effect on the cell viability under normal oxygen levels (referred to as normoxia), it selectively induces programmed cell death in hypoxic cancer cells, thus showing specificity for in vivo tumors. Unlike clinically used autophagy inhibitors that demonstrate general toxicity and activate HIF signaling, we find that NDs selectively inhibit autophagic degradation with minimal impairment of lysosomal functions and do not affect HIF activity. Significantly, intravenous injection of NDs acts synergistically with Sorafenib, an anti-angiogenic drug used clinically for cancer therapy, to inhibit the growth of hepatocellular carcinoma in mice. This distinct mechanism of NDs opens a new door for targeted anti-angiogenic therapy of solid tumors.
- This article is part of the themed collection: Horizons Community Board Themed Collection – Nanobiomedicine

 Please wait while we load your content...
Please wait while we load your content...