Inhibition of α-glucosidase and α-amylase by flavonoid glycosides from Lu'an GuaPian tea: molecular docking and interaction mechanism†
Abstract
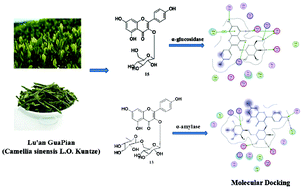
Green tea may favorably modulate blood glucose homeostasis, and regular consumption of green tea can prevent the development of type 2 diabetes mellitus. In this study, α-glucosidase and α-amylase inhibitory effects of the novel acylated flavonol tetraglycoside (camellikaempferoside C, 1) and 14 other flavone and flavone glycosides (FGs) isolated from Lu'an GuaPian (Camellia sinensis L.O. Kuntze) were evaluated. The kaempferol monoglycoside (15) showed inhibitory activity against α-glucosidase with IC50 at 40.02 ± 4.61 μM, and kaempferol diglycoside (13) showed α-amylase inhibition with IC50 at 0.09 ± 0.02 μM. Further, inhibitory mechanisms of FGs 15 and 13 were studied by molecular docking analysis and fluorescence spectrometry. Molecular docking suggested that FG 15 interacted with α-glucosidase mainly by hydrogen bonding, which was the same interaction force between FG 13 and α-amylase. Intrinsic fluorescence of α-glucosidase and α-amylase was quenched by 15 and 13, respectively, through a static quenching mechanism. The spontaneous formation of 15-α-glucosidase and 13-α-amylase complexes was driven by van der Waals forces and hydrogen bonding. The present study provides new insight into the potential application of Lu'an GuaPian green tea as a functional food ingredient to regulate postprandial hyperglycemia through inhibition of α-glucosidase/α-amylase by FGs, particularly the mono− and di− glycosides of kaempferol.


 Please wait while we load your content...
Please wait while we load your content...