Abstract
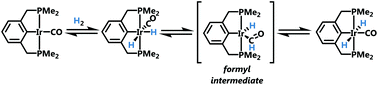
Reduced steric demand of the Me4PCP pincer ligand (PCP = κ3-C6H4-1,3-[CH2PR2]2, R = Me), allows for a more open metal center. This is evident through structure and reactivity comparisons between (Me4PCP)Ir derivatives and other (R4PCP)Ir complexes (R = tBu, iPr, CF3). In particular, isomerization from cis-(R4PCP)Ir(H)2(CO) to trans-(R4PCP)Ir(H)2(CO) is more facile when R = Me than when R = iPr. Deuterium incorporation in the hydride ligands from solvent C6D6 was observed during this isomerization when R = Me. This deuterium exchange has not been observed for other analogous R4PCP iridium complexes. A kinetic study of the cis/trans isomerization combined with computational studies suggests that the cis/trans isomerization proceeds through a migratory-insertion pathway involving a formyl intermediate.
- This article is part of the themed collection: In celebration of Richard Andersen’s 75th birthday


 Please wait while we load your content...
Please wait while we load your content...
