Synthesis and reactivity of an N-triphos Mo(0) dinitrogen complex†
Abstract
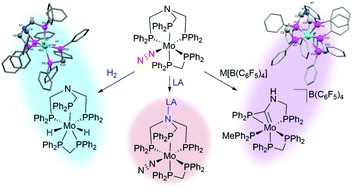
The preparation and reactivity of a novel molybdenum dinitrogen complex supported by a nitrogen-centred tripodal phosphine ligand (N-triphos, N(CH2PPh2)3, NP3Ph) are reported. Reaction of N-triphos with [MoX3(THF)3] (X = Cl, Br, I) gave the Mo(III) complex [MoX3(κ2-NP3Ph)(THF)] (1), where bidentate N-triphos coordination was observed. Reduction of this complex in the presence of dppm (bis(diphenylphosphino)methane) gave the dinitrogen complex [Mo(N2)(dppm)(κ3-NP3Ph)] (2), which exhibits moderate dinitrogen activation. An additional hydride complex, [Mo(H)2(dppm)(κ3-NP3Ph)] (4), was produced either as a minor side product during the reduction step, or as a major product by direct hydrogenation of the dinitrogen complex 2. The reactivity of the dinitrogen complex 2 with a range of Lewis acids was also investigated. At low temperatures, protic or borane Lewis acids (H+, BBr3 and tris(pentafluorophenyl)borane (BCF)) were found to coordinate to the apical nitrogen atom of the N-triphos ligand, with no conclusive evidence of any functionalisation of the dinitrogen ligand. Alkali metal Lewis acid addition to 2 resulted in the unexpected rearrangement of the N-triphos ligand to form [Mo(dppm)(PMePh2)(PCP)][B(C6F5)4] (7), where PCP, [Ph2PCNHCH2PPh2] is the carbenic ligand formed upon rearrangement from the reaction of 2 with M[B(C6F5)4] (M = Li, Na or K). Single crystal X-ray diffraction of complexes 1, 2, 4 and 7 provided structural confirmation of the N-triphos molybdenum complexes described.


 Please wait while we load your content...
Please wait while we load your content...