Chain-propagation, chain-transfer, and hydride-abstraction by cyclic carbocations on water surfaces†
Abstract
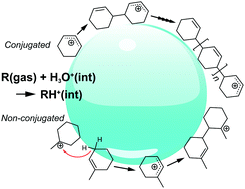
Atmospheric particles contain a wide range of oligomers, but the formation mechanism and the origin of complexity are still unclear. Here, we report the direct detection of carbocationic oligomers generated from the exposure of a series of cyclic unsaturated hydrocarbon gases to acidic water microjets through interface-sensitive mass spectrometry. By changing gas concentrations, H2O (D2O) solvent, bulk pH and comparing results from experiments on acyclic, cyclic, and aromatic compounds, we elucidated three competing reaction mechanisms: chain propagation (CP), chain transfer (CT), and hydride abstraction (HA). We found that conjugative π-electron delocalization in the carbocation is the most important factor for the interfacial oligomerization processes. Our results showed that electrophilic attack on C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds (CP and CT) is limited, and that on C–H single bonds (HA) is enhanced for carbocations lacking conjugation, which is not the case in bulk organic solutions. Carbocationic oligomers generated by the encounter of gaseous unsaturated hydrocarbons and acidic water surfaces potentially contribute to the molecular complexity in atmospheric particles.
C double bonds (CP and CT) is limited, and that on C–H single bonds (HA) is enhanced for carbocations lacking conjugation, which is not the case in bulk organic solutions. Carbocationic oligomers generated by the encounter of gaseous unsaturated hydrocarbons and acidic water surfaces potentially contribute to the molecular complexity in atmospheric particles.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...