X-ray emission spectroscopy: an effective route to extract site occupation of cations†
Abstract
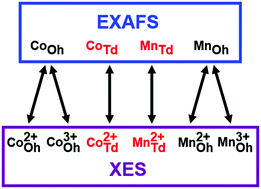
Cation site occupation is an important determinant of materials properties, especially in a complex system with multiple cations such as in ternary spinels. Many methods for extracting the cation site information have been explored in the past, including analysis of spectra obtained through K-edge X-ray absorption spectroscopy (XAS). In this work, we measure the effectiveness of X-ray emission spectroscopy (XES) for determining the cation site occupation. As a test system we use spinel phase CoxMn3−xO4 nanoparticles contaminated with CoO phases because Co and Mn can occupy all cation sites and the impurity simulates typical products of oxide syntheses. We take advantage of the spin and oxidation state sensitive Kβ1,3 peak obtained using XES and demonstrate that XES is a powerful and reliable technique for determining site occupation in ternary spinel systems. Comparison between the extended X-ray absorption fine structure (EXAFS) and XES techniques reveals that XES provides not only the site occupation information as EXAFS, but also additional information on the oxidation states of the cations at each site. We show that the error for EXAFS can be as high as 35% which makes the results obtained ambiguous for certain stoichiometries, whereas for XES, the error determined is consistently smaller than 10%. Thus, we conclude that XES is a superior and a far more accurate method than XAS in extracting cation site occupation in spinel crystal structures.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...
