Microporous mixed-metal mixed-ligand metal organic framework for selective CO2 capture†
Abstract
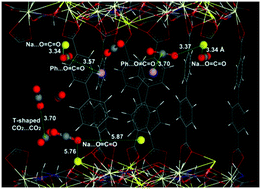
A microporous mixed-ligand-based metal–organic framework has been synthesized using two different dicarboxylic acid-based ligands (4,4′-biphenyldicarboxylate (BPDC) and imino diacetate (IMDA)) and two different metal ions (Ce3+ and Na+), namely Ce3Na3(BPDC)3(IMDA)3·(DMF)2(H2O)9. The framework built from Ce–Na–carboxylate layers and BPDC pillars consists of 2D slit-shaped pores occupied by extra-framework Na+ ions. The desolvated framework is permanently porous with a BET surface area of ∼771 m2 g−1 and displays moderate CO2 uptake of 2.0 mmol g−1 with a CO2/N2 selectivity (S) of 68 at room temperature and 1 bar. A modest heat of adsorption (23 kJ mol−1) and smooth diffusion kinetics are observed, as reflected in the facile CO2 cycling. Using GCMC methods, the CO2 adsorption isotherm at 298 K was simulated, which matches the experimental isotherm well. The CO2 positions observed from the simulations showed that Na+ ions in the channels serve as favorable adsorption sites for the oxygen atoms in CO2 pointing toward the Na+ ions (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯Na+ = 3.34–5.87 Å), while some CO2 molecules sit flat on the phenyl rings of the BPDC at a CO2⋯centroid distance of 3.6–3.7 Å.
O⋯Na+ = 3.34–5.87 Å), while some CO2 molecules sit flat on the phenyl rings of the BPDC at a CO2⋯centroid distance of 3.6–3.7 Å.
- This article is part of the themed collection: CrystEngComm New Talent


 Please wait while we load your content...
Please wait while we load your content...