Understanding the crystalline formation of triazene N-oxides and the role of halogen⋯π interactions†
Abstract
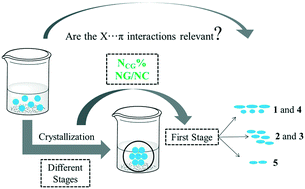
The crystallization of a series of 1-(4-halophenyl)-3-phenyltriazenide N1-oxides (1–4) and 1-(phenyl)-3-phenyltriazenide N1-oxide (5) was evaluated using the supramolecular cluster approach. This method is an efficient tool to assess the crystallization mechanism of compounds and, consequently, the steps involved in crystal formation. Compounds 1 and 4 show crystallization in two main steps while compounds 2 and 3 present three main steps, in which column formation occurs in the first step. The crystallization process for 5 occurs in 3 main steps, starting from a robust dimer formation (−16.82 kcal mol−1). Two new parameters – NCG% (topological and energetic contribution percentage) and NG/NC (energetic parameter/topological parameter ratio) – assisted in the interpretation of crystal growth. Compounds 1–4 showed NCG% = 50 in the first step while compound 5 reached only 50% of the contribution in the second step. The differences in NCG% were attributed to strong hydrogen bonds in the non-halogenated compound. The dominant parameter in each step of the crystallization process was indicated by the NG/NC parameter. The crystallization mechanism in all compounds was initially driven by an energetic process followed by a topological process. The existence of X⋯π interactions was shown and was observed to be a consequence of a topological process and without any major contributions to crystal formation. Thermal analysis and UV-vis spectral data were also discussed regarding the properties of these compounds.


 Please wait while we load your content...
Please wait while we load your content...