Concise syntheses of eburnane indole alkaloids†
Abstract
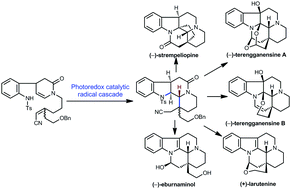
The asymmetric divergent syntheses of a group of C20 ethyl oxo-functionalized eburnane alkaloids, (−)-eburnaminol (5), (+)-larutenine (6), (−)-terengganensine B (7), (−)-strempeliopine (8), and (−)-terengganensine A (9), have been achieved. The key step in the assembly of the complex ring system of the target molecules is a photoredox catalytic nitrogen-centered radical cascade reaction, which allows the regioselective and stereoselective construction of the B, C, and D rings and the installation of the C21 chirality of the eburnane alkaloid skeleton in one pot.


 Please wait while we load your content...
Please wait while we load your content...