Phenylene-bridged cross-conjugated 1,2,3-trisilacyclopentadienes†
Abstract
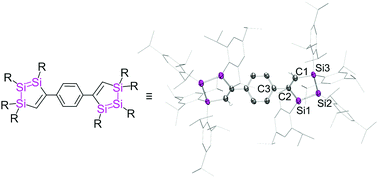
1,2,3-Trisilacyclopentadienes are obtained from the reactions of cyclotrisilene c-Si3R4 (R = iPr3C6H2) with phenyl and diphenyl acetylene, respectively. With 1,4-diethynyl benzene the cross-conjugated bridging of two of the Si3C2 cycles by a para-phenylene linker is achieved. UV/vis spectroscopy indicates a small but significant effect of cross-conjugation, which is confirmed by TD-DFT calculations. The formation mechanism of the 1,2,3-trisilacyclopentadienes is elucidated by VT NMR.


 Please wait while we load your content...
Please wait while we load your content...