Light-mediated iodoperfluoroalkylation of alkenes/alkynes catalyzed by chloride ions: role of halogen bonding†
Abstract
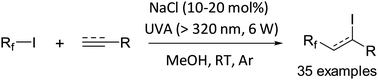
An innovative procedure for the iodoperfluoroalkylation of alkenes/alkynes is described. These reactions, including iodotrifluoromethylations of alkenes, proceed very efficiently under low intensity UVA irradiation in deoxygenated methanol solutions containing catalytic amounts of NaCl or Bu4NCl. Preliminary mechanistic studies indicate that the light-promoted homolytic cleavage of the Rf–I bond that initiates the reaction is facilitated by the halogen bonding interaction between RfI and chloride ions.


 Please wait while we load your content...
Please wait while we load your content...