The role of redox hopping in metal–organic framework electrocatalysis
Abstract
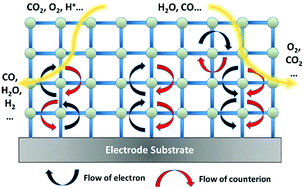
The dominant charge transfer mechanism in a vast number of metal–organic frameworks (MOFs) is that of redox hopping, a process best explained through the motion of electrons via self-exchange reactions between redox centers coupled to the motion of counter-balancing ions. Mechanistic studies of redox hopping transport in MOFs reveal characteristics that recall pioneering studies in linear redox polymers. When MOFs are employed as electrocatalysts, consideration must be given to both the catalytic properties – turn-over frequency (TOF) and energetic requirements (overpotential, TON) – and the charge transport properties – rate of charge hopping, measured via an apparent diffusion coefficient (Dapp). Herein, we provide a mathematical framework to provide constraints to MOF catalyst development by relating Dapp, TOF, and film thickness in the context of providing 10 mA cm−2 of catalytic current. Lastly with the mechanistic studies discussed as a foundation, design rules for future MOF electrocatalysts are provided and the challenges to the community to optimize MOF charge transport are laid out.


 Please wait while we load your content...
Please wait while we load your content...