Towards thermally stable high performance lithium-ion batteries: the combination of a phosphonium cation ionic liquid and a 3D porous molybdenum disulfide/graphene electrode†
Abstract
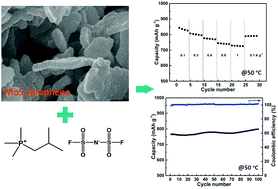
We report a thermally stable high-performance lithium battery using an electrochemically synthesized three-dimensional porous molybdenum disulfide/graphene composite electrode and a phosphonium-based ionic liquid (IL) electrolyte. Benefiting from the structural merits of the chosen electrode and the thermal stability of the electrolyte, the cell coupled with a Li foil exhibits excellent rate performance and cycling capability at room temperature; and that is retained with an even better rate capability at an elevated temperature of 50 °C. This work may provide a new avenue for the development of safe and high performance lithium-ion batteries at high temperature.
- This article is part of the themed collection: Ionic Liquids in the Synthesis, Fabrication, and Utilization of Materials and Devices


 Please wait while we load your content...
Please wait while we load your content...