Light-induced autoxidation of aldehydes to peracids and carboxylic acids†
Abstract
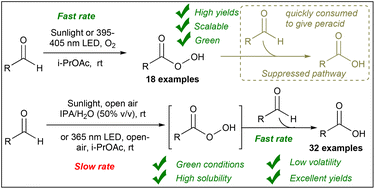
Autoxidation of aldehydes to peracids and carboxylic acids holds a significant impact in both academia and industry due to their wide applications in organic synthesis and environmental remediation. However, the multiple pathways involved in this reaction have hindered the development of sustainable methods for peracid synthesis. Herein, we conduct a comprehensive kinetic and mechanistic investigation to elucidate the interplay between these pathways. Subsequently, we introduce an efficient eco-friendly method to oxidize aldehydes to their corresponding peracids under sunlight or UV irradiation using oxygen as the sole oxidant without any additives or photocatalysts. Additionally, we demonstrate a simple method for the autoxidation of aldehydes to carboxylic acids via controlling key parameters such as the wavelength and solvent. These methods exhibit broad applicability to aromatic and aliphatic aldehydes, successful scaling up to the gram scale, and utilizing renewable solar energy making them good alternatives for traditional methods.


 Please wait while we load your content...
Please wait while we load your content...
