Poly(tetrafluoroethylene-co-perfluorovinyl ether sulfonamide) for anion exchange membranes†
Abstract
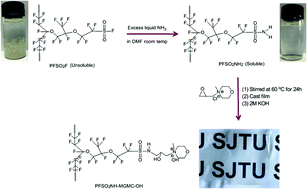
A soluble perfluorinated polymer poly(tetrafluoroethylene-co-perfluorovinyl ether sulfonamide) (PFSO2NH2) was successfully synthesized and used for preparation of perfluorinated anion exchange membranes (AEMs). PFSO2NH2 is soluble in many solvents and has excellent alkaline stability. The perfluorinated AEM (PFSO2NH–MGMC–OH) which was synthesized by the reaction of PFSO2NH2 with 4-methyl-4-glycidylmorpholin-4-ium chloride (MGMC) exhibits a hydroxide conductivity of 60.4 mS cm−1 at 80 °C and 13.8 mS cm−1 at 30 °C, respectively. The transport numbers of the membrane are 0.91 for Cl− and 0.06 for Na+. The membrane shows good alkaline stability that it maintained 80.1% hydroxide conductivity and 85.6% ion exchange capacity (IEC) values after immersion in 8 M KOH over 30 days at 60 °C. This soluble PFSO2NH2 with high thermal and alkaline stability could be used as a precursor for other perfluorinated functional polymers and membranes.

 Please wait while we load your content...
Please wait while we load your content...