Structuring polarity-inverted TBA to G-quadruplex for selective recognition of planarity of natural isoquinoline alkaloids†
Abstract
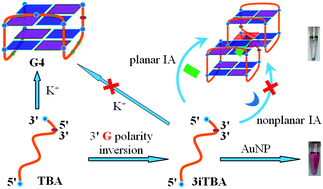
Efficient structuring of DNA by small molecules is very crucial in developing DNA-based novel switches with an ideal performance. In this work, we found that inverting only the polarity of the 3′ terminal guanine of the thrombin-binding aptamer (3iTBA) totally eradicates the original TBA G-quadruplex (G4) structure in K+. The unstructured 3iTBA can be further refolded upon specifically interacting with small molecules of natural isoquinoline alkaloids (IAs) due to their fruitful binding patterns with variant nucleic acid structures. We identified that 3iTBA can serve as a topology selector for planar IAs. Nitidine (NIT), owing to the planar aromatic ring and coplanar substituents, is the most efficient to restructure the 3iTBA random coil toward the anti-parallel G4 conformation. However, common metal ions can't realize this structuring. The topology selector competency of 3iTBA toward IAs’ planarity can be visualized using gold nanoparticles (AuNPs) as the chromogenic readout. Our work expands the G4 repertoire by exploring the polarity inversion regulation and provides a new approach to switch nucleic acid structures toward a small molecule structure-sensitive sensor.
- This article is part of the themed collection: Analyst Recent HOT articles


 Please wait while we load your content...
Please wait while we load your content...