A colorimetric chemosensor for heptanal with selectivity over formaldehyde and acetaldehyde through synergistic interaction of hydrophobic interactions and oxime formation†
Abstract
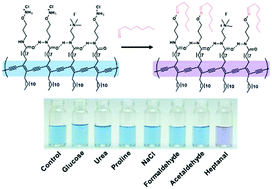
Aldehydes with long alkyl chains are important biomarkers, but chemosensors for the detection of the aldehydes have been rarely reported. Herein, a chemosensor based on hydroxylamine-functionalized polydiacetylene (PDA) was developed for the selective detection of heptanal, which contains a long alkyl chain. The hydroxylamine group of PDA reacts with the aldehyde group of heptanal, while hydrophobic interactions between the alkyl chains of PDA and heptanal occur simultaneously. As a result, synergistic interactions with the aldehyde group and alkyl chain on heptanal allowed for the selective detection of heptanal over formaldehyde and acetaldehyde, which do not contain long alkyl chains. The consequent perturbation of the backbone by the synergistic interactions induced a blue-to-purple color transition, allowing for colorimetric detection by the naked eye. The chemosensor had an estimated detection limit of 4.8 μM. In addition, the sensor system was applied to determine heptanal concentrations in serum samples.
- This article is part of the themed collection: Analyst Recent HOT articles


 Please wait while we load your content...
Please wait while we load your content...