Synthesis and antiviral activity of novel myricetin derivatives containing ferulic acid amide scaffolds†
Abstract
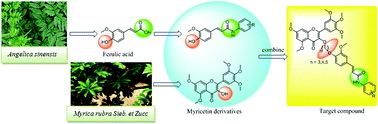
A variety of myricetin derivatives bearing ferulic acid amide scaffolds were designed and synthesized. The structures of all title compounds were determined by 1H NMR, 13C NMR, 19F NMR and HRMS. Preliminary bioassays suggested that some of the target compounds exhibited remarkable antiviral activities. In particular, compound 4l possessed significant protective activity against tobacco mosaic virus (TMV), with a half maximal effective concentration (EC50) value of 196.11 μg mL−1, which was better than that of commercial agent ningnanmycin (447.92 μg mL−1). Meanwhile, microscale thermophoresis (MST) indicated that compound 4l has strong binding capability to the tobacco mosaic virus coat protein (TMV-CP) with a dissociation constant (Kd) value of 0.34 μmol L−1, which was better than that of ningnanmycin (0.52 μmol L−1). These results suggested that novel myricetin derivatives bearing ferulic acid amide scaffolds may be considered as an activator for antiviral agents.


 Please wait while we load your content...
Please wait while we load your content...