Engineering nanoporous Ag/Pd core/shell interfaces with ultrathin Pt doping for efficient hydrogen evolution reaction over a wide pH range†
Abstract
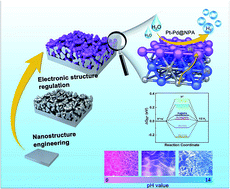
The rational design and fabrication of highly efficient and durable all-pH catalysts for sustainable electrochemical hydrogen production are of critical importance to building renewable energy systems for the future. By employing an in situ electrochemical alloying/dealloying generated nanoporous Ag (NPA) as the supporting substrate, we propose a facile galvanic replacement reaction (GRR) synthesis route in a deep eutectic solvent (Ethaline), combined with an electrochemical activation process to fabricate monolithic 3D nanoporous Ag/Pd core/shell hybrids with ultrathin (sub 1 nm) amorphous Pt-rich skin (Pt–Pd@NPA), showing excellent hydrogen evolution reaction (HER) catalytic performance and durability over a wide pH range. The optimized Pt–Pd@NPA requires low overpotentials of −28.1, −34.8, and −23.8 mV to drive a catalytic current density of −10 mA cm−2 with small Tafel slopes of 31.2, 32.2, and 32.5 mV dec−1 in acidic (0.5 M H2SO4), neutral (1.0 M PBS), and alkaline (1.0 M KOH) media, respectively, which outperforms most previously reported noble-metal-based HER electrocatalysts. Impressively, this hybrid catalyst is capable of steadily delivering a fairly large current density of 1000 mA cm−2 in highly acidic media (0.5–7.0 M H2SO4), promising its practical use in advanced water-splitting devices. The superior HER performance is ascribed to the 3D interconnected nanoporous architectures and synergies between the Ag–Pd skeletons and active Pt. Theoretical calculations confirm that the electronic structure of the Ag–Pd hybrids is optimized by the incorporation of Pt, which results in optimal hydrogen adsorption free energy on the surface and leads to significantly enhanced HER activity and durability. Our work offers a new idea for the design and fabrication of advanced high-performance electrocatalysts for the HER over a wide range of pH values.


 Please wait while we load your content...
Please wait while we load your content...